Sun Pharma and Glenmark locked in trademark tussle
Two big players in the pharmaceutical industry battle it out before the Delhi High Court. The Plaintiff, Sun Pharma Laboratories Limited (Sun Pharma) relying on its rights in the ISTAMET and formative marks has filed the suit against Glenmark Pharmaceuticals Limited (Glenmark) alleging trademark infringement and passing off and to restrain the latter from using the mark INDAMET. This note discusses the contentions raised by the parties and the order passed by the High Court in the injunction application.
Sun Pharma in the suit states:
- They are one of the top pharmaceutical companies in the world and have been engaged in the business since 1978.
- In the year 2010, their predecessor coined and adopted the mark ISTAMET which was assigned to Sun Pharma in July 2022. The medicine sold under the name ‘ISTAMET’ contains the salts, ‘Metformin Hydrochloride’ and ‘Sitagliptin Phosphate Monohyrdrate’, which is used to treat diabetes. It is sold in the form of tablets, under the extensions ISTAMET, ISTAMET XR, and ISTAMET XR CP, and is a Schedule ‘G’ drug. The mark ISTAMET XR CP is registered in class 5 since 2014.
- In May 2022, Sun Pharma noticed Glenmark’s application for the mark INDAMET in class 5 which was duly opposed, and the proceedings are ongoing before the Trademark Registry. Glenmark’s further application for the mark INDA-MET in class 5 is pending registration.
- Although the applications for the marks INDAMET and INDA-MET have been filed on a ‘proposed to be used’ basis, inquiries revealed sales of INDAMET on online pharma websites.
- Sun Pharma’s ‘ISTAMET’,’ISTAMET XR’ and ‘ISTAMET XR CP” products are extensively sold and have acquired goodwill and reputation in the market.
- INDAMET is visually, structurally and phonetically similar to Sun Pharma’s ISTAMET XR CP and amounts to infringement and passing off.
Glenmark counters the injunction application on the following:
- Sun Pharma is not entitled to injunction as they have not disclosed the settlement proposal made by Glenmark with regard to limiting the specification of goods.
- In June 2022, they launched ‘INDAMET’ which is a novel fixed dose combination drug for uncontrolled asthma.
- ‘INDAMET’ is distinguishable from the mark ‘ISTAMET”/ ‘ISTAMET XR CP’, as both are for different ailments. INDAMET is sold as a capsule to be inhaled with a dry-powder inhaler whereas, ISTAMET is a tablet. The products will not be sold over the counter and the differences in packaging will avoid any chances of confusion.
- The mark ‘ISTAMET’ is not registered and the registration for the mark ‘ISTAMET XR CP’ must be read as a whole, inclusive of the terms ‘XR’ and ‘CP’. Thus, no monopoly can be claimed over the mark ‘ISTAMET’ more so qua all goods under Class 5 considering that registration for ‘ISTAMET XR CP’ is limited to preparations for diabetes. The marks ‘INDAMET’ and ‘ISTAMET XR CP’ are not visually, structurally and phonetically similar.
- INDAMET has been coined by deriving ‘INDA’ from the chemical compound ‘Indacaterol acetate” and ‘MET’ from ‘Mometasone furorate’. Thus, Sun Pharma cannot claim any monopoly over the term ‘MET’ as it is common to the trade.
- Sun Pharma has taken an inconsistent and contradictory stand regarding INDAMET as opposed to the cited marks in its own registrations. For the mark ‘ISTAMET XR CP’, Sun Pharma, distinguished its mark from one of the cited conflicting mark ‘INTAMET’, as dissimilar. Further, Sun Pharma’s device mark
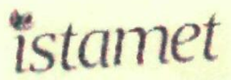 stands refused by Registrar of Trademarks due to prior registered marks in Class 5 including ‘ASTAMET’, ‘INSTAMET’ and ‘ESTIMET’. Sun Pharma had distinguished its device mark vis-à-vis the cited marks in its response to the Examination Report, as dissimilar. Thus, Sun Pharma, in the present suit, cannot be permitted to approbate and reprobate.
stands refused by Registrar of Trademarks due to prior registered marks in Class 5 including ‘ASTAMET’, ‘INSTAMET’ and ‘ESTIMET’. Sun Pharma had distinguished its device mark vis-à-vis the cited marks in its response to the Examination Report, as dissimilar. Thus, Sun Pharma, in the present suit, cannot be permitted to approbate and reprobate. - With regard to the concerns relating to public health, if a person suffering from diabetes takes ‘INDAMET’, which is used for treatment of asthma, instead of ‘ISTAMET’, there will be no negative impact and the drug would just pass through the system.
After considering the pleadings, arguments and case laws the Court held:
- Glenmark’s contention that the rival marks ISTAMET XR CP and INDAMET compared as a whole are not similar will not succeed. Though Sun Pharma’s registration is for the composite mark ‘ISTAMET XR CP’, the dominant feature is the word ‘ISTAMET’. Moreover, the terms ‘XR’ (denoting ‘extended release’) and ‘CP’ (indicating ‘combipack’), is commonly used by pharmaceutical companies to describe products. The argument that Sun Pharma cannot claim monopoly on the suffix ‘MET’ ought to fail as the Court is not dissecting the mark for comparing the suffix selectively. The rival marks ‘ISTAMET’ and ‘INDAMET’ compared as a whole are structurally and phonetically similar.
- The argument that the rival products will be sold only on prescription has no significance. Considering the overall similarity between the marks, the likelihood of confusion for a buyer cannot be ruled out merely because the packaging is different. Confusion regarding method of administration can lead to potential health risks and the deceptive similarity of the marks could lead to confusion at the point of purchase irrespective of the mode of administration.
- The specificity outlined in Sun Pharma’s registration confining the pharmaceutical product to be utilized for diabetes, ought not to be interpreted narrowly. The argument that Sun Pharma cannot claim monopoly over the term ‘MET’ has no relevance as the marked similarity between the rival brand names overshadow these differences in composition, due to the shared suffix ‘MET.’ It is also relevant to note that, in pharmaceuticals, minor differences in composition can yield varied results including potential side effects.
- Glenmark’s contention that accidental ingestion of INDAMET would not cause any harm is unfounded. It is not supported by any scientific evidence, study, or peer reviewed publication to indicate the potential consequences of inadvertent consumption of the rival medications. Thus, the clinical effects of wrong consumption of an incorrect drug is a grey area and cannot be a point of differentiation to rule out any confusion between the rival drugs. Moreover, the method of administration cannot serve as the sole differentiator for the products and holds little significance in the instant case.
- The argument that Sun Pharma had at the time of registration distinguished the ISTAMET mark from conflicting marks INTAMET, INSTAMET and ESTIMET and is thus, estopped from contesting similarity with INTAMET mark ought not to succeed. The distinction drawn at the time of registration in relation to third party conflicting marks has no relevance to the mark at hand. Thus, any stance taken at the time of registration or opposition with regard to third party marks cannot be logically applied to the INDAMET mark. Legal rights carry significant weight and cannot be decided on the basis of responses to examination report or opposition in unrelated matters.
- Considering that Sun Pharma has been using the mark since 2011 and established market recognition balance of convenience is in their favour. Glenmark’s decision to proceed with the INDAMET mark despite the pending opposition evidences their willingness to risk potential legal outcomes.
In light of the above, the Court granted an injunction in favour of Sun Pharma restraining Glenmark from using the mark INDAMET or any other mark deceptively similar to ISTAMET XR CP.
Glenmark has assailed the order of the Single Judge before the Division Bench (DB, Two Judge Bench). The DB has admitted the appeal and stayed the Single Judge’s order, and the appeal proceedings are ongoing before the Delhi High Court.



