Delhi High Court examines factors for the patentability of species patent in India
In a landmark decision, the Delhi High Court denied an injunction to the Plaintiff (BOEHRINGER INGELHEIM PHARMA GMBH) for infringement of patent rights in Linagliptin, a drug to treat Type 2 diabetes. While deciding the infringement action, the Court analyzed various vital factors, including species versus genus patent infringement. In this case, the Court also looked at the evergreening of the Patent, public interest, and prior claiming.
Linagliptin is sold under the brand name Trajenta, which acts by inhibiting the enzyme dipeptidyl peptidase-4 (DPP-4). Unlike other DPP-4 inhibitors, linagliptin is excreted chiefly via the enterohepatic system, and safe for patients with renal or hepatic impairment. In 2020, it was the 293rd most prescribed medication in the United States, with more than 1 million prescriptions. From August 2021, linagliptin became available as a generic medicine in the U.S. The company is not manufacturing the drug in India. It has licensed the suit Patent to Lupin Laboratories and Eli Lilly, for which royalties are payable by the said entities to the plaintiffs.
Background and facts:
The Plaintiff, BOEHRINGER INGELHEIM PHARMA GMBH, Patentee of two patents- IN’227719 i.e., genus patent (IN ‘719) and IN’243301 i.e., species patent (IN ‘301), filed six suits against various Indian pharmaceutical companies seeking an injunction against the manufacturing of Linagliptin. Due to the common issues involved, the Court has given a joint decision in the six suits filed against various defendants.
Genus patents are broad patents covering a group of potential products. Genus claims are written mainly by Markush structure, which claims a large class of chemical compounds. At the same time, the species/selection patent covers a specific compound, which forms a part of the Markush structure claimed in the genus patent. Simply put, the genus patent has a broader coverage, while the species patent has a specific coverage.
The Plaintiff invoked genus patent (IN ‘719) and species patent (IN ‘301) in two of the six suits i.e., C.S. (COMM) 239/2019 and C.S. (COMM) 240/2019 against Vee Excel Drugs and Pharmaceuticals Private Ltd.
The genus patent (IN ‘719) expired on February 21st 2022, while the species patent (IN ‘301) will expire on August 18th, 2023. After the expiry of genus patent (IN ‘719), the Plaintiff filed four suits i.e., C.S. (COMM) 236/2022, C.S. (COMM) 237/2022, C.S. (COMM) 238/2022 and C.S. (COMM) 296/2022, seeking an injunction against the Defendants (Indian Pharmaceutical companies). The Defendants in turn, challenged the validity of the species patent (IN ‘301), on the grounds of section 3(d) and prior claiming as their defence.
According to Section 3(d), the mere discovery of a new form of a known substance that does not result in the enhancement of the known efficacy of that substance is not patentable. It means that different forms of a known substance must differ significantly in the properties regarding efficacy. Overall, the legislature’s intention by including section 3(d) was to disallow ever-greening of patents.
The ground of prior claiming is raised where the invention (first application) claimed in a complete specification filed in India has already been claimed in the complete specification of an application (second application) which was filed in India before the priority date of the first application but published on or after the filing of the second application.
The Court formulated the following main issues for the grant of interim injunction in favour of the plaintiffs:
Prior claiming
- The Court opined that a comparison, in the below table, shows that a substantial part of the chemical structure in Claim 1 of the suit patent and the genus patent are structurally similar. Considering that the plaintiffs have themselves in the proceedings before the Controller admitted that Linagliptin is one of the possible substitutions of IN ‘719, it would leave no matter of doubt that both the patents are attempting to cover the same subject matter as well. This would not be permissible under the Patents Act.
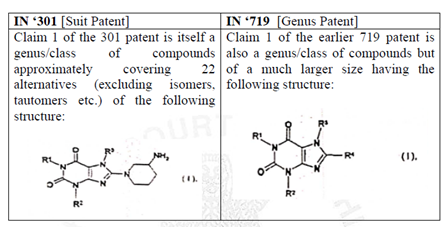
- The Court also found indications of prior claiming not just in the suit Patent. Still, there were such indications even during the International Phase of prosecuting the suit Patent. The International Search Report (ISR) issued concerning the PCT publication of the species patent by the International Search Authority mentioned the corresponding PCT publication of the genus patent IN‘719, i.e., W.O. 02/068420 as a P and X (for determination of novelty and inventive step in regional/national procedures). In India, considering that prior claiming is a ground for revocation under Section 64(1)(a) of the Patents Act, 1970, the P, X reference document highlighted by the ISA is striking at the novelty and inventive step of the species patent.
- While responding to the examination regarding the genus patent application, Plaintiff submitted a list of 371 compounds, one of which was Linagliptin. The Court noted that the plaintiffs specifically claimed Linagliptin to obtain the grant of the genus patent. However, in the rejoinder filed on behalf of the plaintiffs to I.A. 5806/2022, the plaintiffs made a complete U-turn and stated that Linagliptin had not been claimed in IN ‘719.
Data in Form 27 / Working statement:
After perusing the two Form 27s of the same period for genus and the species patent, the Court found that all the pharmaceutical products which the two patents claim to be working in India are the same. In addition, the quantum and value of imports and sales are also the same. Therefore, it is evident that both IN ‘301 and IN ‘719 are directed toward the same invention, which is not permissible as per Section 10 of the Act.
Evergreening:
The Court noted that by filing multiple patents for different aspects of the same product, the Plaintiffs are seeking to extend the term of the Patent beyond twenty years, granted in respect of the genus patent. Since, the Patent expired on February 21st, 2022, it amounts to evergreening or layering of patent protection, which is impermissible under the Indian Patent Law under Section 3(d) of the Patents Act. The Court referred the findings from Novartis v. Union of India, (2013) 6 SCC 1 (Novartis) for section 3(d) where the Supreme Court dealt with the issue of whether the therapeutic drug, beta crystalline form of Imatinib Mesylate, qualifies as an invention under Section 2(1)(j) and Section 2(1)(ja) of the Patents Act and whether the Patent can be refused under Section 3(d) of the Patents Act, highlighting that “Under the scheme of Patent, a monopoly is granted to a private individual in exchange of the invention being made public so that, at the end of the patent term, the invention may belong to the people at large who may be benefited by it. To say that the coverage in a patent might go much beyond the disclosure thus seem to negate the fundamental rule underlying the grant of patents.”
Balance of Convenience:
The Court addressed the following issues about the balance of convenience and irreparable injury, the fundamental principles which govern the grant of interim injunction.
- Whether balance of convenience is in favour of the plaintiffs and against the defendants for the grant of interim injunction?
- Whether the plaintiffs would suffer irreparable injury on account of non-grant of interim injunction?
The Court, while examining the above two questions, noted that the plaintiffs have enjoyed a twenty-year monopoly of Linagliptin under the genus patent. Except for the defendants in C.S. (COMM) 239/2019 and C.S. (COMM)240/2019, the other defendants waited for the twenty-years term of the genus patent to expire on February 21st, 2022, before launching their drugs in the market.
Further, the plaintiffs do not manufacture their drugs in India, but import their drugs into India. Clearly, the intention of the plaintiffs is to monetise the said invention.
Therefore, the present case is one where monetary damage can be calculated and awarded to the plaintiffs in the event the plaintiffs succeed in the present suits. It is a settled position of law that where monetary damages are the adequate compensation for the plaintiffs, an interim injunction should not be granted.
Public interest:
The Court thus considered the elements of public interest as the drug Linagliptin is used for the treatment of diabetes, which is a widely prevalent disease in India. Therefore, the public interest also demands that large segments of the population should have easy and affordable access to an anti-diabetes drug. Undeniably, the defendant’s products are significantly cheaper than that of the plaintiffs. Considering that Linagliptin is a daily-use drug, affordability plays a major role in its access to wide sections of the public.
Thus, finally taking all the above factors into consideration, the Court concluded that prima facie suit patent of the plaintiffs, i.e., IN‘301 is vulnerable to revocation on the ground of prior claiming in terms of Section 64(1)(a) of the Patents Act, as the plaintiffs have made an attempt towards evergreening the invention and re-monopolizing the same.
Summary:
The order in the present case highlights that the courts also considered the factor of “public interest” equally as to the patentability / technical parameters, while deciding an injunction/ infringement proceedings at the interim stage, particularly in the pharmaceutical matters.
The public interest defence has often been pushed as a “one size fits all” approach in every pharma patent infringement matter and in this case as well it was a strong argument that the injunction will not only affect the interest of the infringer but also the end consumer.
For the species patent to get approval over the teaching/ disclosure of genus patent, it is important to show technical advancement or enhancement of the known efficacy to qualify the objection of section 3(d). Ideally, there should not be any overlap between disclosure and claims in the patent specifications for genus and species patent.
The plaintiff has filed appeal before the Division Bench of the High court challenging this order. The appeal is yet to be heard on merits. It will be interesting to see the outcome of the appeal bench on the patentability of Linagliptin in the species patent in view of the above-mentioned facts.
The above factors were also discussed in an earlier case AstraZeneca AB & Anr. v. Intas Pharmaceuticals Ltd., where the Delhi High court dealt with Dapagliflozen (‘DAPA’) which is useful for the treatment of type -II diabetes mellitus. DAPA was claimed in the subject matter of two Patents:
- IN205147 (‘IN 147’) discloses Markush structure i.e. a group of compounds that covers ‘DAPA’ and is a genus Patent.
- No.235625 (‘IN 625’) specifically discloses DAPA and is a species Patent. The Patent is valid until May 5th 2023.
AstraZeneca, based on these two patents, had sued several Indian pharmaceutical companies for patent infringement. Here, the Court observed that a single formulation as DAPA, is incapable of protection under two separate patents having separate validity period. When the inventor is the same, the tests regarding “obvious to a person skilled in the art”, “anticipation by publication” and “use before the date of filing of patent application with complete specification”, cannot be in the context of “person ordinarily skilled in the art” but have to be of the “person in the know”. It was also mentioned by the Court that the applicant cannot enjoy two rounds of 20 years of protection, when the legislative policy is to grant protection for a period of one term of 20 years only.
In another recent case, on March 23rd, the Indian Patent Office also rejected Janssen’s secondary (or species) patent application on the fumarate salt form of Bedaquiline, which is a drug for the treatment of multi-drug resistant tuberculosis. The Controller refused the patent application on the basis that the pharmaceutical composition of base compound Bedaquiline against M. tuberculosis was already covered under the patents previously granted in favour of applicant. The controller also mentioned in the order that the applicant had to show data on how an increase in bioavailability results in increased therapeutic efficacy. The combination of fumarate salt of Bedaquiline along with common pharmaceutically acceptable excipients wetting agent is considered as known substance and not patentable u/s 3(d) of the Act. Further, it was also found that the application verbatim copied several portions particularly, the portions related to the use of a wetting agent, from Janssen’s own patent application.
Key takeaways:
- The genus patent may cover multiple chemical compounds, however, only those compounds will be considered as disclosed which can be obtained by going through the disclosure of the genus patent.
- The species patent application should have enhanced therapeutic efficacy over the compound of genus patent to overcome section 3(d) requirement.
- Further, as noted from the facts of the above-mentioned cases, the Patentee needs to be cautious of the submissions being made in the response to the examination report, working of the patent information filed in Form 27 and other documents filed during the prosecution of genus and species patent. There should not be the overlapping disclosure/s in the complete specification/s and the information in official documents needs to be consistent.



